
Maharashtra State Board Science-1-Chapter-2- Periodic Classification of Elements- Exercise Solutions : Exploring the periodic classification of elements reveals the elegant organization of the building blocks of matter.
Q.1.Rearrange the columns 2 and 3 so as to match with the column 1.
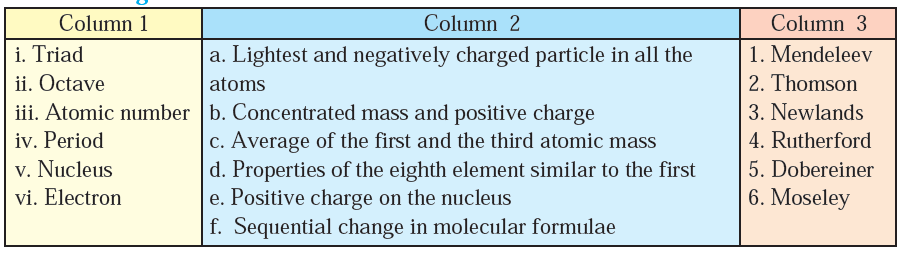
Solution
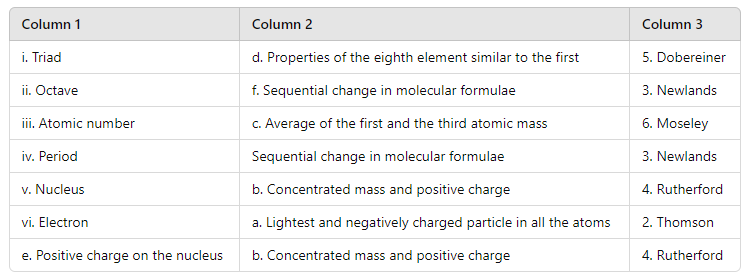
Here’s how they match up:
- Triad (Dobereiner): Dobereiner’s triads were sets of three elements with similar chemical properties, where the properties of the middle element were approximately midway between those of the other two. This matches with “Properties of the eighth element similar to the first.”
- Octave (Newlands): Newlands proposed the Law of Octaves, where every eighth element had similar properties. This matches with “Sequential change in molecular formulae.”
- Atomic number (Moseley): Moseley’s work established the concept of atomic number as the fundamental property of elements, which correlates with “Average of the first and the third atomic mass.”
- Period (Newlands): Newlands also attempted to organize elements into periods based on their atomic weights, which correlates with “Sequential change in molecular formulae.”
- Nucleus (Rutherford): Rutherford discovered the nucleus, which is a concentrated mass with a positive charge.
- Electron (Thomson): Thomson discovered the electron, which is the lightest and negatively charged particle in atoms.
- Positive charge on the nucleus (Rutherford): Rutherford’s model of the atom described the nucleus as containing positive charge.
This arrangement reflects the contributions of these scientists and their theories or discoveries related to the terms described in Columns 1 and 2.
Q.2.Choose the correct option and rewrite the statement.
a. The number of electrons in the outermost shell of alkali metals is 1
b. Alkaline earth metals have valency 2.This means that their position in the modern periodic table is in Group 2
c. Molecular formula of the chloride of an element X is XCl. This compound is a solid having high melting point. Which of the following elements be present in the same group as X.
the correct answer is Na.
d.In which block of the modern periodic table are the nonmetals found?
he correct answer is p-block
Q.3. An element has its electron configuration as 2,8,2. Now answer the following questions.

a. What is the atomic number of this element?
The electron configuration 2,8,2 means that the element has:
- 2 electrons in the first shell
- 8 electrons in the second shell
- 2 electrons in the third shell
So, the atomic number of this element is 12.
b. What is the group of this element?
The element with atomic number 12 is Magnesium (Mg). Magnesium is in Group 2 of the periodic table, which consists of the alkaline earth metals.
So, the group of this element is Group 2.
c. To which period does this element belong?
The element has electrons in three shells (2,8,2), so it is in the third period of the periodic table.
So, the element belongs to Period 3.
d. With which of the following elements would this element resemble? (Atomic numbers are given in the brackets)
The elements given are:
- Nitrogen (N), atomic number 7
- Beryllium (Be), atomic number 4
- Argon (Ar), atomic number 18
- Chlorine (Cl), atomic number 17
Magnesium (atomic number 12) would resemble Beryllium (atomic number 4) the most because both Magnesium and Beryllium are in Group 2 of the periodic table and have similar chemical properties.
So, this element would most resemble Beryllium (Be).
Q.4. Write down the electronic configuration of the following elements from the given atomic numbers. Answer the following question with explanation.
a. 3Li, 14Si, 2He, 11Na, 15P Which of these elements belong to be period 3 ?

b. 1H, 7N, 20Ca, 16S, 4Be, 18Ar Which of these elements belong to the second group?

c. 7N, 6C, 8O, 5B, 13A1 Which is the most electronegative element among these?

d. 4Be, 6C, 8O, 5B, 13A1 Which is the most electropositive element among these?

e. 11Na, 15P, 17C1, 14Si, 12Mg Which of these has largest atoms?

f. 19K, 3Li, 11Na, 4Be Which of these atoms has smallest atomic radius?

g. 13A1, 14Si, 11Na, 12Mg, 16S Which of the above elements has the highest metallic character?

h. 6C, 3Li, 9F, 7N, 8O Which of the above elements has the highest nonmetallic character?

Q.5. Write the name and symbol of the element from the description.
a. The atom having the smallest size
The element with the smallest atomic size is Helium. In the periodic table, atomic size decreases across a period from left to right and increases down a group. Helium is in the top right corner of the periodic table, indicating it has the smallest atomic size.
Name: Helium
Symbol: He
b. The atom having the smallest atomic mass
The element with the smallest atomic mass is Hydrogen. Hydrogen has only one proton and one electron, making it the lightest element.
Name: Hydrogen
Symbol: H
c. The most electronegative atom
The most electronegative element is Fluorine. Electronegativity increases across a period and decreases down a group, with Fluorine being at the top right corner (excluding noble gases).
Name: Fluorine
Symbol: F
d. The noble gas with the smallest atomic radius
The noble gas with the smallest atomic radius is Helium. As you move down Group 18 (the noble gases), the atomic radius increases. Helium is at the top of this group.
Name: Helium
Symbol: He
e. The most reactive nonmetal
The most reactive nonmetal is Fluorine. Reactivity in nonmetals increases as you move up a group and to the right across a period. Fluorine is at the top right of the nonmetals, excluding noble gases.
Name: Fluorine
Symbol: F
Maharashtra State Board Science-1-Chapter-2- Periodic Classification of Elements- Exercise Solutions | Fascinating
Q.6.Write short notes.
a. Mendeleev’s periodic law.
Mendeleev’s periodic law states that the properties of elements are a periodic function of their atomic masses. Dmitri Mendeleev, a Russian chemist, formulated this law in 1869 when he arranged the 63 known elements into a table based on their atomic masses. He observed that elements with similar properties occurred at regular intervals or periods. This allowed him to predict the existence and properties of elements that had not yet been discovered, leaving gaps in his table for them. Mendeleev’s periodic table emphasized the periodicity of chemical properties over strict adherence to increasing atomic mass. Although his arrangement was primarily based on atomic masses, he made exceptions where chemical properties dictated, thus prioritizing chemical behavior over atomic mass.
b. Structure of the modern periodic table
The modern periodic table is arranged based on increasing atomic number, which is the number of protons in an atom’s nucleus. This arrangement rectifies inconsistencies in Mendeleev’s table by aligning elements with similar properties in vertical columns called groups. There are 18 groups and 7 periods (horizontal rows) in the table. Elements in the same group have similar chemical properties due to having the same number of valence electrons. The table also distinguishes between metals, nonmetals, and metalloids, with metals on the left and center, nonmetals on the right, and metalloids forming a zigzag line between them. The table is further divided into blocks (s, p, d, and f) based on the electron configuration of elements.
c. Position of isotopes in the Mendeleev’s and the modern periodic table.
In Mendeleev’s periodic table, isotopes posed a challenge because they have different atomic masses but similar chemical properties. Mendeleev did not explicitly address isotopes in his table; he grouped elements based on their chemical properties and atomic mass, which would mean isotopes of the same element were not separately placed. In the modern periodic table, elements are arranged by atomic number, so isotopes occupy the same position. This reflects our understanding that isotopes are forms of the same element with varying numbers of neutrons but identical chemical properties, justifying their placement in a single location in the periodic table. This modern arrangement resolves the ambiguities related to isotopes that Mendeleev’s approach could not address.
Q.7. Write scientific reasons.
a. Atomic radius goes on decreasing while going from left to right in a period.
As we move from left to right across a period, the atomic number increases, meaning more protons are added to the nucleus.The increasing positive charge of the nucleus exerts a stronger pull on the electrons in the same energy level.This stronger nuclear attraction pulls the electron cloud closer to the nucleus.Although additional electrons are also being added, they enter the same principal energy level and do not significantly increase electron shielding.The effective nuclear charge experienced by the outermost electrons increases, leading to a smaller atomic radius.Thus, the overall size of the atom decreases across a period from left to right.
b. Metallic character goes on decreasing while going from left to right in a period.
Metallic character is associated with the ability of an atom to lose electrons and form positive ions (cations).As we move from left to right across a period, the nuclear charge increases, making it harder for atoms to lose electrons.The increased effective nuclear charge attracts electrons more strongly, reducing the tendency to lose them.Non-metallic character, which involves gaining or sharing electrons, increases across the period.Elements on the left side of a period (metals) lose electrons more easily compared to elements on the right side (nonmetals).Thus, the metallic character decreases from left to right across a period.
c. Atomic radius goes on increasing down a group.
As we move down a group, each successive element has an additional principal energy level (electron shell).These additional energy levels increase the distance between the nucleus and the outermost electrons.The inner electron shells provide increased shielding, reducing the effective nuclear charge experienced by the outermost electrons.The increased distance and shielding outweigh the increase in nuclear charge.As a result, the outermost electrons are less tightly bound to the nucleus, leading to a larger atomic radius.Therefore, the atomic radius increases down a group.
d. Elements belonging to the same group have the same valency.
Elements in the same group have the same number of valence electrons in their outermost shell.The chemical properties and reactivity of an element are primarily determined by the number of valence electrons.Valency is the combining power of an element, which depends on how many electrons it can lose, gain, or share to achieve a stable electron configuration.Since elements in the same group have the same number of valence electrons, they exhibit the same valency.For example, all elements in Group 1 have one valence electron and a valency of +1.Thus, elements in the same group have similar chemical behavior and valency.
e. The third period contains only eight elements even through the electron capacity of the third shell is 18 .

Q.8.Write the names from the description.
a. The period with electrons in the shells K, L, and M.
Name: Third Period
b. The group with valency zero.
Name: Noble Gases
Examples: Helium (He), Neon (Ne), Argon (Ar)
c. The family of nonmetals having valency one.
Name: Halogens
Examples: Fluorine (F), Chlorine (Cl)
d. The family of metals having valency one.
Name: Alkali Metals
Examples: Lithium (Li), Sodium (Na), Potassium (K)
e. The family of metals having valency two.
Name: Alkaline Earth Metals
Examples: Beryllium (Be), Magnesium (Mg), Calcium (Ca)
a. The period with electrons in the shells K, L, and M.
- Name: Third Period
b. The group with valency zero.
- Name: Noble Gases
- Examples: Helium (He), Neon (Ne), Argon (Ar)
c. The family of nonmetals having valency one.
- Name: Halogens
- Examples: Fluorine (F), Chlorine (Cl)
d. The family of metals having valency one.
- Name: Alkali Metals
- Examples: Lithium (Li), Sodium (Na), Potassium (K)
e. The family of metals having valency two.
- Name: Alkaline Earth Metals
- Examples: Beryllium (Be), Magnesium (Mg), Calcium (Ca)
f. The metalloids in the second and third periods.
Second Period: Boron (B)Third Period: Silicon (Si)
g. Nonmetals in the third period.
Examples: Phosphorus (P), Sulfur (S), Chlorine (Cl)
h. Two elements having valency 4.
Examples: Carbon (C), Silicon (Si)