Maharashtra State Board Science-1-Chapter-2-Periodic Classification of Elements-Can You Recall? : The concept of periodic classification of elements is fundamental to understanding the organization and behavior of elements in chemistry. This classification system arranges elements based on their properties and allows scientists to predict their chemical behavior and relationships with other elements.
The periodic table, a visual representation of this classification, groups elements with similar characteristics into columns known as groups or families. Elements within the same group often share similar chemical properties due to their identical number of electrons in their outermost energy levels.
Dmitri Mendeleev, a Russian chemist, is credited with the initial development of the periodic table in the late 19th century. His arrangement was based on atomic mass and predicted the existence of undiscovered elements based on gaps in the table. Later, Henry Moseley’s work established that the periodic table should be organized by atomic number, which is the number of protons in an atom’s nucleus.
Understanding the periodic table is crucial for students of chemistry as it provides a systematic framework for organizing and studying the vast array of elements that compose matter in the universe. This chapter delves into the structure of the periodic table, the trends observed across periods and groups, and how this organization helps in understanding the behavior and properties of elements.
Can You Recall?
What are the types of matter?
Matter can generally be classified into several types based on its physical properties and structure. The most common classification includes
- Solid : Solids have a definite shape and volume. The particles in solids are closely packed together and vibrate in place. Examples include ice, wood, and metal.
- Liquid : Liquids have a definite volume but take the shape of their container. The particles in liquids are close together but can move past each other. Examples include water, oil, and milk.
- Gas : Gases have neither a definite shape nor volume. They expand to fill their container completely. The particles in gases are far apart and move freely. Examples include air, oxygen, and carbon dioxide.
- Plasma : Plasma is a state of matter where the gas phase is energized until atomic electrons are no longer associated with any particular atomic nucleus. Plasma is the most abundant form of matter in the universe, occurring in stars, including the sun, and other celestial bodies.
- Bose-Einstein Condensate (BEC): This is a state of matter formed at temperatures close to absolute zero. In this state, a group of boson particles (e.g., certain isotopes of helium and other atoms) behaves as if they were a single particle. BEC exhibits quantum phenomena on a macroscopic scale.
These are the primary states of matter commonly recognized in science, each with distinct characteristics based on the arrangement and behavior of its particles.
What are the types of elements?
Elements are substances that cannot be broken down into simpler substances by ordinary chemical means. They are composed of atoms with the same number of protons in their atomic nuclei. Elements can be classified into several types based on their properties and positions in the periodic table. Here are the main types of elements:
- Metals: Metals are typically solid (except for mercury) and have good conductivity of electricity and heat. They are usually shiny, malleable (can be hammered into thin sheets), and ductile (can be drawn into wires). Examples include iron, copper, gold, and aluminum.
- Nonmetals: Nonmetals can be found in all three states of matter (solid, liquid, gas). They are generally poor conductors of electricity and heat, and they are not shiny. Nonmetals can be brittle and lack the characteristic properties of metals. Examples include carbon, sulfur, oxygen, and nitrogen.
- Metalloids (Semimetals): Metalloids have properties that are intermediate between metals and nonmetals. They exhibit characteristics of both groups to varying degrees. Metalloids are typically semiconductors of electricity, meaning their conductivity can be controlled. Examples include silicon, germanium, and arsenic.
What are the smallest particles of matter called?
The smallest particles of matter are called atoms. Atoms are the basic building blocks of all matter in the universe. They are incredibly small, with diameters typically ranging from about 0.1 to 0.5 nanometers (nm).
Atoms are composed of even smaller subatomic particles:
- Protons: Positively charged particles found in the nucleus of an atom.
- Neutrons: Neutral particles found in the nucleus of an atom.
- Electrons: Negatively charged particles that orbit the nucleus in specific energy levels or shells.
The nucleus of an atom (composed of protons and neutrons) is surrounded by a cloud of electrons. The number of protons in the nucleus determines the element’s identity (e.g., hydrogen, oxygen, carbon), while the number of neutrons and electrons can vary, leading to different isotopes and ions of the same element.
Atoms combine to form molecules, which are the smallest units of chemical compounds. The study of atoms and their interactions forms the basis of chemistry and our understanding of the physical world.
What is the difference between the molecules of elements and compounds?
The difference between molecules of elements and compounds lies in their composition and the types of atoms they contain:
- Molecules of Elements:
- Molecules of elements consist of atoms of the same element chemically bonded together.
- These molecules are formed when atoms of the same element share electrons to achieve a stable electron configuration.
- Molecules of elements can exist as diatomic molecules (two atoms bonded together) or in other forms depending on the element’s structure and bonding properties.
- Molecules of Compounds:
- Molecules of compounds consist of atoms of different elements chemically bonded together.
- These molecules are formed when atoms of different elements combine through chemical bonds to achieve a stable electron configuration.
- Molecules of compounds are characterized by the specific ratios in which atoms of different elements are combined according to the compound’s chemical formula.
In summary, molecules of elements are composed of atoms of the same element bonded together, while molecules of compounds are composed of atoms of different elements bonded together. Understanding the distinction between these two types of molecules is essential in chemistry for identifying substances and understanding their properties and behaviors.

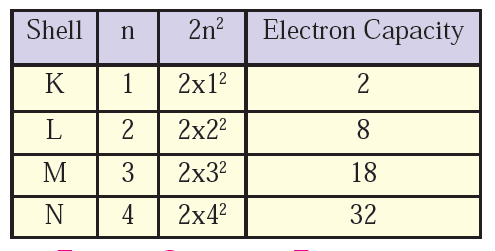
What are the values of ‘n’ for the shells K, L and M?
The shells in an atom are designated by letters, starting from K and extending outward alphabetically (K, L, M, N, etc.). These letters correspond to the principal quantum number ( n ), which represents the energy level or shell of an electron in an atom.
For the shells K, L, and M:
- K shell: ( n = 1 )
- The K shell is the innermost shell in an atom. It can hold up to 2 electrons.
- L shell: ( n = 2 )
- The L shell is the second shell from the nucleus. It can hold up to 8 electrons.
- M shell: ( n = 3 )
- The M shell is the third shell from the nucleus. It can hold up to 18 electrons.
These values of ( n ) indicate the principal quantum number for each respective shell. As you move outward from the nucleus of an atom, the shells increase in size and energy level, allowing them to accommodate more electrons according to the rules of atomic structure.
What is the maximum number of electrons that can be accommodated in a shell? Write the formula.
The maximum number of electrons that can be accommodated in a shell of an atom can be determined using the formula based on the principal quantum number ( n ).
The formula to calculate the maximum number of electrons in a shell is:
[ 2n^2 ]
Where:
- ( n ) is the principal quantum number representing the shell number.
Let’s apply this formula to a few examples:
- For K shell (( n = 1 )):
[ 2 x 12 = 2 ]
- The K shell can accommodate a maximum of 2 electrons.
- For L shell (( n = 2 )):
[ 2 x 22 = 2 x 4 = 8 ]
- The L shell can accommodate a maximum of 8 electrons.
- For M shell (( n = 3 )):
[ 2 x 32 = 2 x 9 = 18 ]
- The M shell can accommodate a maximum of 18 electrons.
This formula ( 2n2 ) is derived from the fact that each principal energy level or shell can hold up to 2 electrons per orbital (where each orbital corresponds to a specific subshell within the shell). Therefore, the total number of electrons that can fit into a shell is based on the number of orbitals available within that shell, which increases with the square of the principal quantum number ( n ).
Deduce the maximum electron capacity of the shells K, L and M.