Science-1-Chapter-3- Chemical Reactions And Equations- Can You Tell? : Chemical reactions are at the heart of chemistry, transforming substances into new forms through the breaking and forming of bonds. Equations represent these changes, providing a precise language to describe what happens during a reaction. Whether you’re a student new to chemistry or a seasoned scientist, understanding the intricacies of chemical reactions and equations is crucial. In this post, we’ll dive into the fundamental concepts, explore common types of reactions, and learn how to balance equations, ensuring a solid foundation in this essential aspect of science. Let’s get started on this fascinating journey into the world of chemical transformations!
Q.1. Take into account the time required for following processes. Classify them into two groups and give titles to the groups.
- Cooking gas starts burning on ignition .
- Iron article undergoes rusting.
- Erosion of rocks takes place to form soil.
- Alcohol is formed on mixing yeast in glucose solution under proper condition.
- Effervescence is formed on adding baking soda into a test tube containing dilute acid.
- A white precipitate is formed on adding dilute sulphuric acid to barium chloride solution.
Rapid and Slow Reactions: Understanding the Pace of Chemical Processes
Chemical reactions can vary greatly in the time they take to complete. Some reactions occur almost instantaneously, while others can take years or even centuries. Here, we’ll classify various reactions into two categories based on their speed: Rapid Reactions and Slow Reactions.
Rapid Reactions
- Cooking gas starts burning on ignition
- Description: This is a combustion reaction that happens almost instantly when the gas is ignited.
- Example: Ignition of LPG (liquefied petroleum gas) in a stove.
- Effervescence is formed on adding baking soda into a test tube containing dilute acid
- Description: This reaction is immediate and produces carbon dioxide gas, causing effervescence.
- Example: Reaction between sodium bicarbonate (baking soda) and acetic acid (vinegar).
- A white precipitate is formed on adding dilute sulphuric acid to barium chloride solution
- Description: This is a precipitation reaction that occurs rapidly, forming barium sulfate as a white solid.
- Example: Reaction between sulfuric acid and barium chloride solution.
Slow Reactions
- Iron article undergoes rusting
- Description: Rusting is a slow oxidation process that takes place over a long period.
- Example: Formation of iron oxide on an iron fence over months or years.
- Erosion of rocks takes place to form soil
- Description: This is a very slow geological process that can take thousands to millions of years.
- Example: Weathering of rocks by wind, water, and biological activity.
- Alcohol is formed on mixing yeast in glucose solution under proper condition
- Description: This is a fermentation process that takes several hours to days to complete.
- Example: Fermentation of glucose to ethanol by yeast in brewing.
By understanding whether a reaction is rapid or slow, we gain insights into the nature of the processes involved and can better control and utilize them in various applications, from industrial manufacturing to daily household activities.
Chapter-3- Chemical Reactions And Equations- Can You Tell?
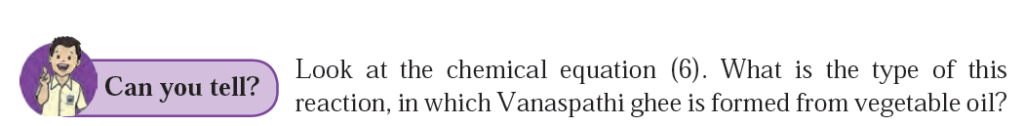
Q.2. Look at the chemical equation (6). What is the type of this reaction, in which Vanaspathi ghee is formed from vegetable oil?
The chemical equation given is:
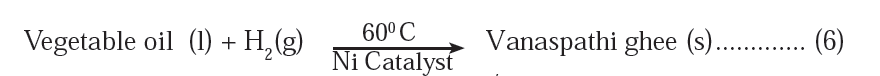
Explanation:
Hydrogenation is a chemical reaction that involves the addition of hydrogen (H₂) to another compound. In this specific case, hydrogen is added to vegetable oil to convert it into Vanaspathi ghee.
Characteristics of Hydrogenation:
- Type of Reaction: Addition reaction, specifically the addition of hydrogen.
- Catalyst: Typically, this reaction requires a catalyst such as nickel, palladium, or platinum to proceed efficiently.
- Change in State: The reaction converts unsaturated fats (present in vegetable oil) to saturated fats (present in Vanaspathi ghee), which results in the change from a liquid to a semi-solid or solid state.
This process is widely used in the food industry to increase the shelf life of oils and to convert liquid oils into a more solid form, suitable for various culinary uses.