Maharashtra State Board Science-1-Chapter-2-Periodic Classification of Elements-Use Your Brain Power? | Fascinating : Unlocking the potential of our brain is key to mastering any subject, including those covered in the Class 10 textbook. By tackling ‘Use Your Brain Power’ questions, students not only enhance their problem-solving skills but also deepen their understanding of core concepts. Let’s delve into how these questions challenge us to think critically and creatively, paving the way for academic success and cognitive growth.
1) Chlorine has two isotopes,viz, C1-35 and C1-37 Their atomic masses are 35 and 37 respectively.Their chemical properties are same. Where should these be placed in Mendeleev’s periodic table? In different places or in the same place?
In Mendeleev’s periodic table, chlorine isotopes Cl-35 and Cl-37 would be placed in the same group (or column). This is because isotopes of an element have the same number of protons and electrons, hence they exhibit the same chemical properties. The only difference between Cl-35 and Cl-37 is the number of neutrons in their nuclei, which affects their atomic masses but not their chemical behavior.
Therefore, both Cl-35 and Cl-37 would be placed in the same group as chlorine (Group 17, also known as Group VIIA in some older notation) in Mendeleev’s periodic table.
2) Write the molecular formulae of oxides of the following elements by referring to the Mendeleev’s periodic table. Na, Si, Ca, C, Rb, P, Ba, Cl, Sn.
molecular formulas of oxides for each element based on their position in Mendeleev’s periodic table:
1. Na (Sodium) – Sodium forms sodium oxide:
– Molecular formula: Na2O
2. Si (Silicon) – Silicon forms silicon dioxide (also known as silica):
– Molecular formula: SiO2
3. Ca (Calcium) – Calcium forms calcium oxide (also known as lime or quicklime):
– Molecular formula: CaO
4. C (Carbon) – Carbon forms several oxides, the most common are carbon monoxide and carbon dioxide:
– Molecular formulas:
– Carbon monoxide: CO
– Carbon dioxide: CO2
5. Rb (Rubidium) – Rubidium forms rubidium oxide:
– Molecular formula: Rb2O
6. P (Phosphorus) – Phosphorus forms two main oxides, phosphorus(III) oxide and phosphorus(V) oxide:
– Molecular formulas:
– Phosphorus(III) oxide (or phosphorus trioxide): P4O6
– Phosphorus(V) oxide (or phosphorus pentoxide): P4O10
7. Ba (Barium) – Barium forms barium oxide:
– Molecular formula: BaO
8. Cl (Chlorine)- Chlorine forms several oxides, including chlorine dioxide and dichlorine monoxide:
– Molecular formulas:
– Chlorine dioxide: ClO2
– Dichlorine monoxide: Cl2O
9. Sn (Tin) – Tin forms tin(II) oxide and tin(IV) oxide:
– Molecular formulas:
– Tin(II) oxide (or stannous oxide): SnO
– Tin(IV) oxide (or tin dioxide or stannic oxide): SnO2
These molecular formulas correspond to the typical oxides formed by these elements based on their positions and chemical properties in Mendeleev’s periodic table.
3) Write the molecular formulae of the compounds of the following elements with hydrogen by referring to the Mendeleev’s periodic table. C, S, Br, As, F, O, N, Cl
Here are the molecular formulas of compounds formed by each element with hydrogen, based on their positions in Mendeleev’s periodic table:
- C (Carbon) – Carbon forms several compounds with hydrogen, known as hydrocarbons:
- Molecular formulas:
- Methane: CH4
- Ethane: C2H6
- Propane: C3H8
- Butane: C4H10
- And so on, following the general formula CnH(2n+2) for alkanes.
- S (Sulfur) – Sulfur forms hydrogen sulfide:
- Molecular formula: H2S
- Br (Bromine) – Bromine forms hydrogen bromide:
- Molecular formula: HBr
- As (Arsenic) – Arsenic forms arsine:
- Molecular formula: AsH3
- F (Fluorine) – Fluorine forms hydrogen fluoride:
- Molecular formula: HF
- O (Oxygen) – Oxygen forms water:
- Molecular formula: H2O
- N (Nitrogen) – Nitrogen forms ammonia:
- Molecular formula: NH3
- Cl (Chlorine) – Chlorine forms hydrogen chloride:
- Molecular formula: HCl
These molecular formulas represent common compounds formed by these elements with hydrogen, reflecting their positions and chemical properties in Mendeleev’s periodic table.
4) How is the problem regarding the position of cobalt (59Co) and nickel (59Ni) in Mendeleev’s periodic table resolved in modern periodic table?
In Mendeleev’s original periodic table, the placement of cobalt (59Co) and nickel (59Ni) posed a challenge because their atomic weights were very close, leading to ambiguity in their correct positions. Both elements were known to exhibit similar chemical properties, which further complicated their classification.
In the modern periodic table, developed after significant advancements in understanding atomic structure and properties, the issue of placing cobalt and nickel was resolved primarily by organizing elements based on their atomic numbers rather than atomic weights or chemical similarities alone. Here’s how the modern periodic table resolves this:
- Based on Atomic Number: The modern periodic table arranges elements in order of increasing atomic number (number of protons in the nucleus of an atom). This fundamental change from Mendeleev’s table, which was based on atomic weight and chemical properties, ensures that elements with similar chemical behaviors due to their electronic configurations are grouped together.
- Electronic Structure: Cobalt (59Co) and nickel (59Ni) have atomic numbers 27 and 28, respectively. In the periodic table:
- Cobalt (Co) is placed in period 4, group 9 (transition metals).
- Nickel (Ni) is placed in period 4, group 10 (transition metals).
- Grouping and Properties: Despite their close atomic weights and similar chemical properties, nickel is consistently placed after cobalt in the modern periodic table due to its higher atomic number. This organization reflects their electronic configurations, where nickel has one more proton and electron compared to cobalt, influencing its chemical behavior and placement in the periodic table.
In summary, the modern periodic table resolves the issue by organizing elements based on atomic number, a more fundamental property of elements than atomic weight or chemical similarities alone. This approach ensures that cobalt (59Co) and nickel (59Ni) are correctly placed according to their atomic numbers, reflecting their distinct positions in the periodic table based on their electron configurations and chemical behaviors.
5) How did the position of 17C135 and 17C137 get fixed in the modern periodic table?
The elements you’re referring to, 135Cesium (135Cs) and 137Cesium (137Cs), are isotopes of the element cesium (Cs). Their positions in the modern periodic table are not fixed in the traditional sense of the periodic table layout (groups and periods), but rather they are categorized based on their atomic number and properties within the broader context of cesium.
Here’s how their position is understood in the modern context:
- Atomic Number and Identification: Both 135Cs and 137Cs are isotopes of cesium (Cs). Isotopes have the same number of protons (and thus the same atomic number), but different numbers of neutrons, leading to different atomic masses.
- Atomic Number and Periodic Table Positioning: Cesium (Cs) itself has an atomic number of 55, placing it in period 6 and group 1 (alkali metals) of the periodic table. This placement is based on cesium’s atomic number, which is the defining factor in the modern periodic table.
- Isotopic Abundance and Applications: In practical terms, 135Cs and 137Cs are important due to their isotopic properties, especially in the context of nuclear physics, radiation, and environmental monitoring (137Cs in particular is a significant radioactive isotope resulting from nuclear fallout). They are classified as cesium isotopes rather than separate elements with unique positions in the periodic table.
- Usage and Classification: In scientific and technical literature, these isotopes are commonly referred to by their mass numbers (135 and 137) appended to the element symbol (Cs), emphasizing their isotopic nature rather than treating them as distinct elements with separate positions in the periodic table.
Therefore, the positions of 135Cs and 137Cs are not fixed in terms of traditional periodic table groups and periods but are understood within the context of cesium (Cs) based on their atomic numbers and isotopic properties. This reflects the modern approach where isotopes are identified by their element symbol followed by their mass number, acknowledging their relationship to the element’s atomic structure.
6) Can there be an element with atomic mass 53 or 54 in between the two elements, chromium 52Cr24 and manganese 25Mn25 ?
No, there cannot be an element with atomic mass 53 or 54 between chromium (24Cr) and manganese (25Mn) in the modern periodic table. Here’s why:
- Atomic Number Sequence: In the periodic table, elements are arranged in order of increasing atomic number (number of protons in the nucleus), not atomic mass. Chromium (24Cr) has an atomic number of 24, and manganese (25Mn) has an atomic number of 25.
- Atomic Mass Variation: While the atomic masses of isotopes of elements can vary (due to different numbers of neutrons), the position of elements in the periodic table is primarily determined by their atomic number. Elements with atomic numbers between 24 (chromium) and 25 (manganese) must have atomic numbers that follow this sequence without skipping.
- Elemental Placement: There are no naturally occurring elements with atomic numbers 24.5, 24.6, etc. Each element occupies a specific position in the periodic table based on its atomic number. Chromium is in period 4, group 6 (transition metals), and manganese is in period 4, group 7 (transition metals).
- Isotopes vs. Elements: Isotopes of an element, which have different atomic masses due to varying numbers of neutrons, do not constitute separate elements. They are variations of the same element and do not disrupt the sequential order of elements in the periodic table.
Therefore, any hypothetical element with an atomic mass of 53 or 54 would either not exist or would be a synthetic element not yet discovered or confirmed. In the context of the modern periodic table, the positions of elements like chromium and manganese are fixed based on their atomic numbers, and no elements with atomic numbers between 24 and 25 exist in nature.
7) What do you think? Should hydrogen be placed in the group 17 of halogens or group 1 of alkali metals in the modern periodic table ?
In the modern periodic table, hydrogen is placed separately from both group 1 (alkali metals) and group 17 (halogens). Here’s why:
- Properties of Hydrogen:
- Hydrogen is unique because it exhibits properties that can resemble both alkali metals (group 1) and halogens (group 17) under different conditions.
- Like alkali metals, hydrogen has one valence electron and can lose it to form H⁺ ions (protons).
- Like halogens, hydrogen can gain one electron to form H⁻ ions (hydrides).
- Placement in the Periodic Table:
- Hydrogen is typically placed above group 1 in the periodic table, but it is not part of group 1.
- Its position reflects its unique nature and the fact that it does not fit neatly into either the alkali metals or halogens due to its diverse range of chemical behaviors.
- Variability in Compounds:
- Hydrogen forms a wide variety of compounds with elements across the periodic table, not just with elements in group 1 or group 17.
- Its compounds include hydrides (like NaH, LiH), which are more akin to those of alkali metals, and hydrogen halides (like HCl, HF), which are more akin to those of halogens.
- Hydrogen as a Bridge Element:
- Hydrogen is sometimes considered a bridge between metals and non-metals due to its ability to form metallic hydrides (with characteristics similar to metals) and covalent compounds (with characteristics similar to non-metals).
In conclusion, while hydrogen shares some characteristics with both alkali metals and halogens, its unique properties and the diversity of its chemical behaviors necessitate its separate placement in the periodic table. Therefore, in the modern periodic table, hydrogen is not placed in either group 1 (alkali metals) or group 17 (halogens); instead, it stands alone at the top of the table as a distinctive element with its own group.
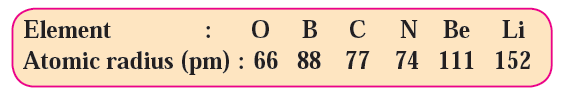
8) By referring to the modern periodic table find out the periods to which the above elements belong.
Periods of the Elements:
- Oxygen (O) belongs to period 2.
- Boron (B) belongs to period 2.
- Carbon (C) belongs to period 2.
- Nitrogen (N) belongs to period 2.
- Beryllium (Be) belongs to period 2.
- Lithium (Li) belongs to period 2.
Therefore, all these elements belong to period 2 of the modern periodic table.
9) Arrange the above elements in a decreasing order of their atomic radii.
Decreasing Order of Atomic Radii:
- Lithium (Li) = 152 pm
- Beryllium (Be) = 111 pm
- Boron (B) = 88 pm
- Carbon (C) = 77 pm
- Nitrogen (N) = 74 pm
- Oxygen (O) = 66 pm
Therefore, the elements arranged in decreasing order of their atomic radii are:
- Lithium (Li)
- Beryllium (Be)
- Boron (B)
- Carbon (C)
- Nitrogen (N)
- Oxygen (O)
10) Does this arrangement match with the pattern of the second period of the modern periodic table?
Matching with Period 2 Pattern:
- In period 2 of the modern periodic table, the trend in atomic radii generally decreases from left to right across the period. This is because as you move from left to right across a period, the number of protons in the nucleus increases, leading to a greater nuclear charge pulling the electrons closer to the nucleus, thereby decreasing the atomic radius.
- The arrangement of atomic radii we have (Li > Be > B > C > N > O) matches this trend where atomic radii generally decrease from left to right within a period.
11) Which of the above elements have the biggest and the smallest atom?
Biggest and Smallest Atom:
- The element with the biggest atom (largest atomic radius) among the listed elements is Lithium (Li).
- The element with the smallest atom (smallest atomic radius) among the listed elements is Oxygen (O).
13) What is the periodic trend observed in the variation of atomic radius while going from left to right within a period?
Periodic Trend in Atomic Radius Across a Period:
- The atomic radius generally decreases from left to right across a period in the periodic table.
- This trend occurs because as you move across a period, the number of protons in the nucleus increases, which increases the nuclear charge. This greater nuclear charge attracts the electrons more strongly, pulling them closer to the nucleus and thus reducing the atomic radius.
- Exceptions to this trend can occur due to electron configuration changes (e.g., the atomic radius of Oxygen is smaller than Nitrogen despite being to the right in period 2, likely due to the filling of the p-orbital and repulsion between electrons in the same shell).